Features of ELISA platform
Long shelf life and excellent stability.
More particularly, we can offer 24+ months shelf life of ready to use calibrators across all ELISA assays.
Generalized Substrates and Stop Solutions across all ELISA assays.
Ready to use and colorized TMB Substrate has the features of high titer and good stability.
With excellent stability of Stop Solution, there will be no sediments occurring after stopping, and more importantly no difference and variation during 3-hour detections.
Features of CMIA platform
CMIA = Chemiluminescent Microparticle Immuno Assay
wo solid phase carrier platform technical services for Biostreptomycin system and FITC system are available. All products can be designed and developed as a solid phase carrier on each platform.
Two major substrate systems, including horseradish (HRP) and acridinium ester, are available. At the same time, all the Washing Solutions and Substrates can be generalized for use.
Based on the above platforms, product can be developed under the principle— such as sandwich method, competition method, indirect method, and capture method.
Rapid launch to market
Bringing in years of specialist experience and a proven development approach, our dedicated team of professionals will help you market your assay fast and cost-effectively. Your assay will be developed in compliance to ISO 13485 and you will receive the complete Design History file from us.
We are the partner for in vitro diagnostic (IVD) assay development – for start-ups and mid-sized biotech companies in the global IVD medical device market. With 15+ years of expert experience and 100+ IVD developments, our enthusiastic and highly skilled professionals are committed to accelerating healthcare innovation, providing high-quality assay development and manufacturing services.
Whether you need highly specialized knowledge or manpower to bring your concept to market, we are your trusted independent partner for any type of immunoassay development using any type of technology on any type of analyzer.
Immunoassays development
In addition to developing complete IVD immunoassays on demand, we also offer flexible support in pre-feasibility, design & verification and production and validations projects. Our professional and experienced experts will help your R&D team accelerate and bring your immunoassay to market rapidly.
When developing an immunoassay, what we do, in essence, is choose a format, gather the right components, and construct a good working protocol. Every immunoassay format provides a different level of specificity, sensitivity, simplicity and speed, and requires different numbers and varieties of components.
To ensure assay test results are robust and accurate, we will systematically adjust and test all components and variables in order to optimize the assay.
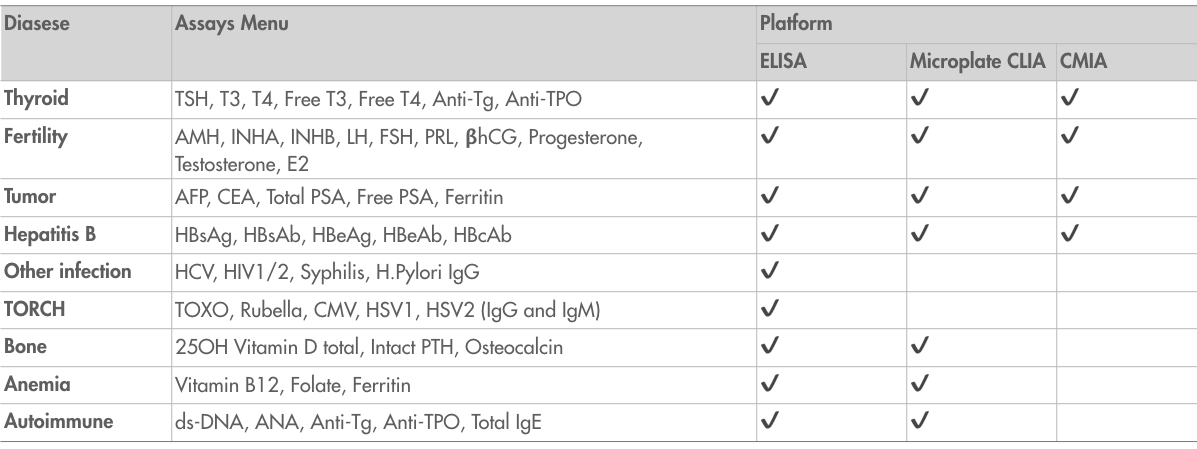
List of developed assays
We have successfully developed a wide variety of immunoassays that help clinicians diagnose and monitor diseases, and manage and improve patient outcomes.
© 2024 DiaSino Laboratories Co., Ltd. All Rights Reserved.